Will Vaxart’s oral COVID vaccine candidate show at least 75% efficacy in a human challenge trial?
Plus
12
Ṁ2831Jan 1
6%
chance
1D
1W
1M
ALL
Background: Vaxart is planning to run a human challenge trial using the Omicron strain of COVID to test its oral vaccine candidate. See: https://investors.vaxart.com/news-releases/news-release-details/vaxart-announces-agreement-hvivo-develop-worlds-first-human
Resolves YES if the reported efficacy against infection is 75% or more, NO if under 75% efficacy, and N/A if the vaccine trial winds up not happening, or if efficacy against infection isn’t used as a clinical endpoint (although that seems unlikely).
This question is managed and resolved by Manifold.
Get 1,000and
1,000and 3.00
3.00
Sort by:
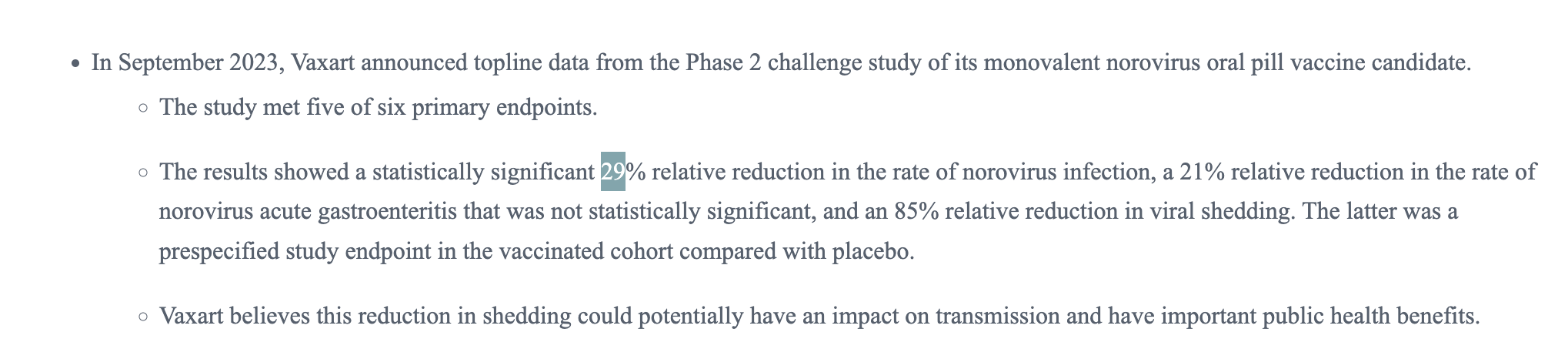
looks like it's gonna resolve NO
https://investors.vaxart.com/news-releases/news-release-details/vaxart-provides-business-update-and-reports-third-quarter-2023
Related questions
Related questions
Will the VLA15 Lyme Disease vaccine "pass" its phase 3 human trial?
61% chance
Will an H5N1 mRNA vaccine be developed and enter "late stage" human trials (or wider release) by July 2025?
47% chance
If a human challenge trial for a hepatitis C vaccine is completed by the end of 2024, will a hep C vaccine be approved by the end of 2027?
53% chance
Will the M72/AS01E tuberculosis vaccine candidate have an efficacy of 50% or higher in the phase 3 trial?
59% chance
If a human challenge trial for a hepatitis C vaccine is NOT completed by the end of 2024, will a hep C vaccine be approved by the end of 2027?
46% chance
Will an mRNA HIV vaccine pass Phase 2 trials by 2025
21% chance
Will there be a successful human trial of a vaccine for rheumatoid arthritis by the end of 2028?
22% chance
Will the HTVN 142 clinical trial to vaccinate against HIV be successful?
81% chance
What will be the vaccine efficacy of the two-dose Johnson & Johnson Ad26.COV2.S vaccine candidate according to the results of Phase III testing?
85% chance