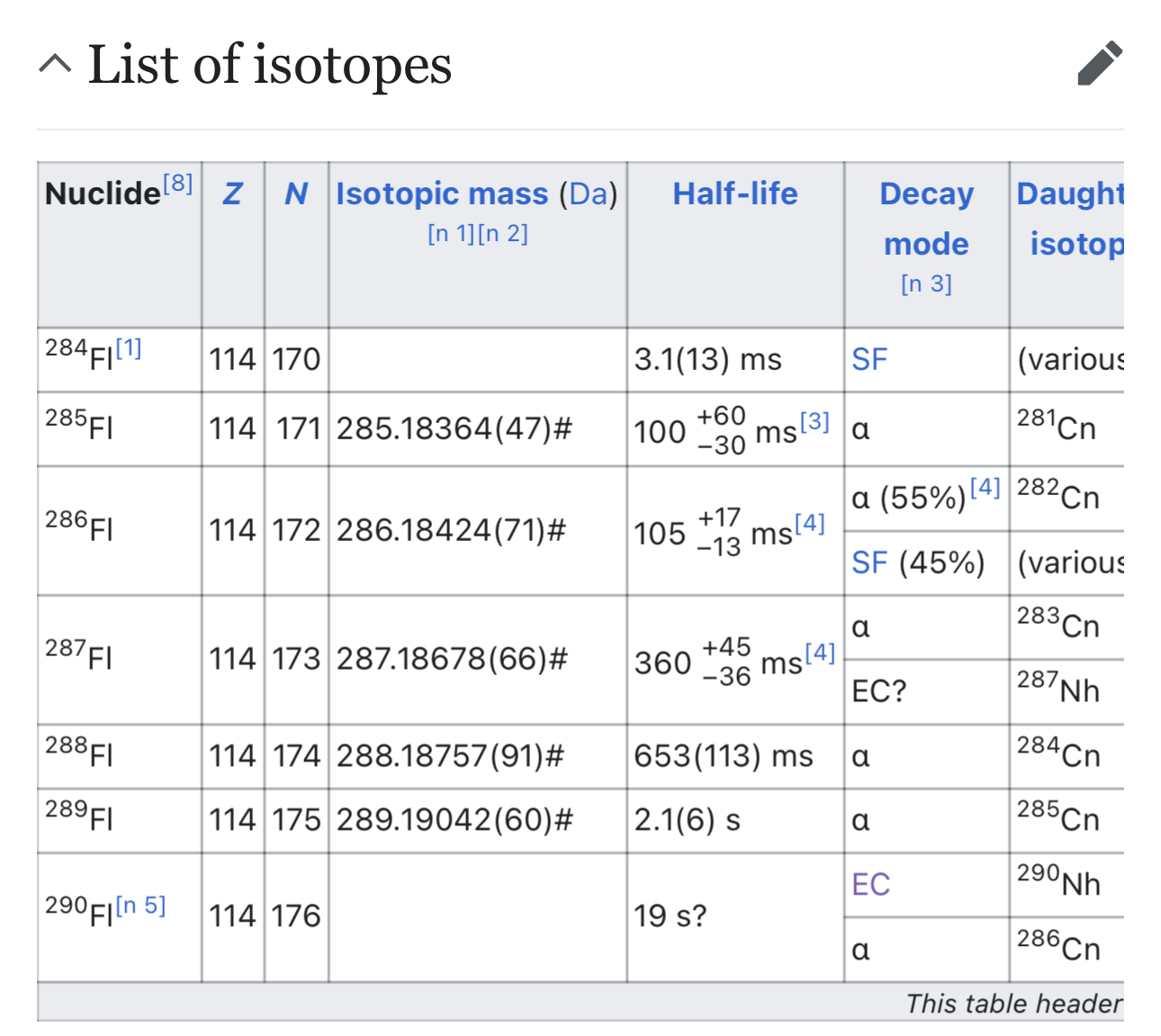
This isotope is doubly magic with Z=114 and N=184 therefore it’s predicted to be much longer lived than other superheavy isotopes https://en.m.wikipedia.org/wiki/Island_of_stability
Resolves PROB to 10x the base-10 logarithm of the half life in seconds. So 1s =0%, 10s=10%, 100s=20% and so on.
Resolves whenever the half-life is determined experimentally to within +/- 30%
This might never occur due to the difficulty of synthesis.
I'm skeptical it'll make it to anything like second lengths. There are some energetic enough cosmic events nearby that we'd've probably been able to observe a glimmer of its lines if it were stable enough to stay together for sufficient absorption/re-emission interactions. I think somebody did a survey for looking for superheavies not too long ago.
@JonathanRay But it’s just really hard to produce because the n/p ratio is so much higher than any of the source materials they could work with
@JonathanRay I'm a computational chemist. I could tell you it'll outlast the heat death and technically be an expert opinion. But, in my actual somewhat expert opinion, I think we'd've certainly seen more evidence of superheavies in nature if they had stabilities on the order of hours plus.
That doesn't mean I don't think they exist at all but I'd be quite surprised if they made it past the second mark.
Primordial heavies are made by the r-process of rapidly absorbing neutrons, but the probability of getting all the way to 298 by that process without a fission is basically zero. Lab syntheses of superheavy nuclei are always colliding other heavy nuclei in accelerators (which requires absurdly high energies and results in n/p ratios way too low)
@AndrewHartman haven’t other isotopes been confirmed to have half lifes in the seconds (some claims up to 10s of seconds)